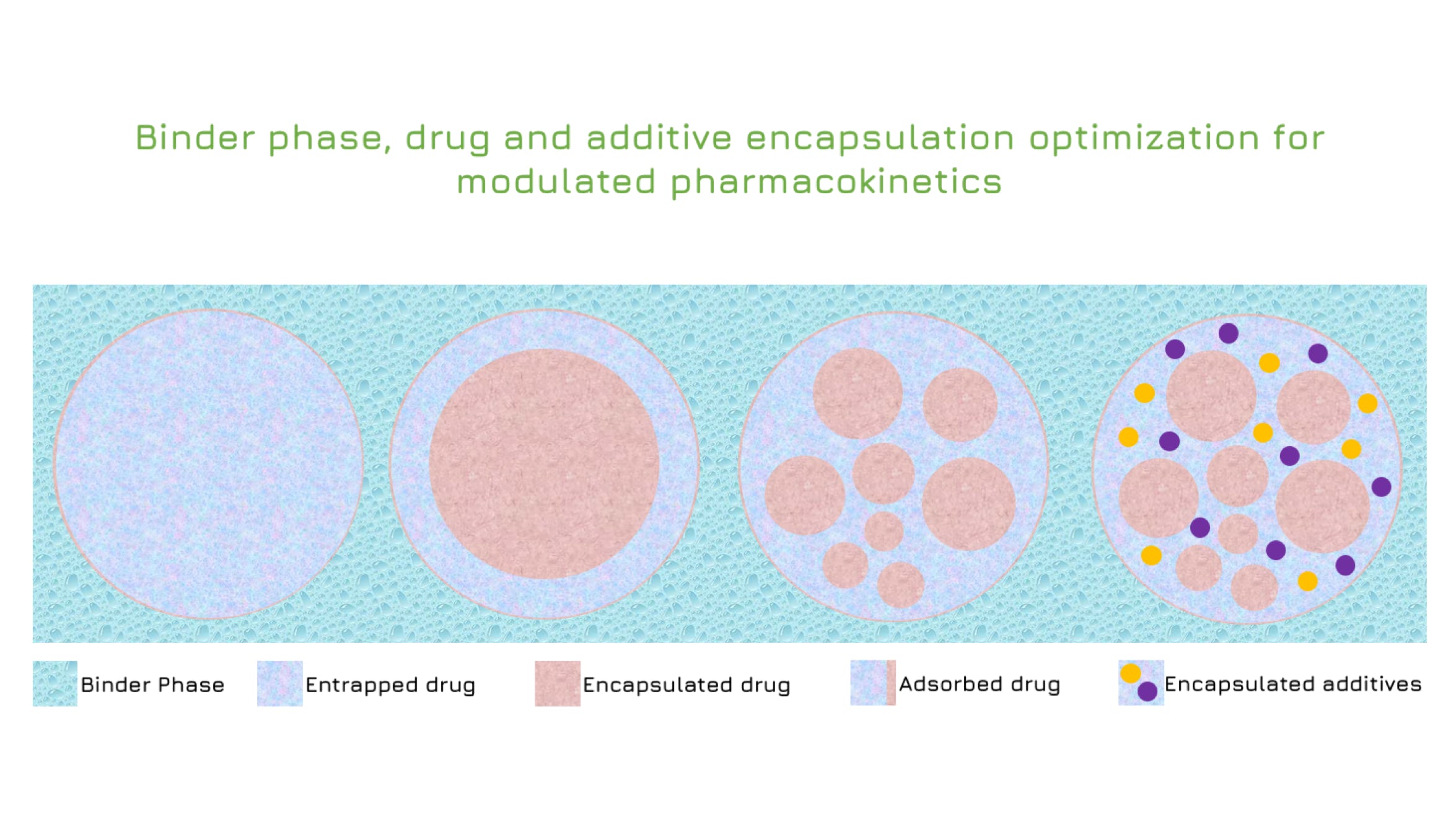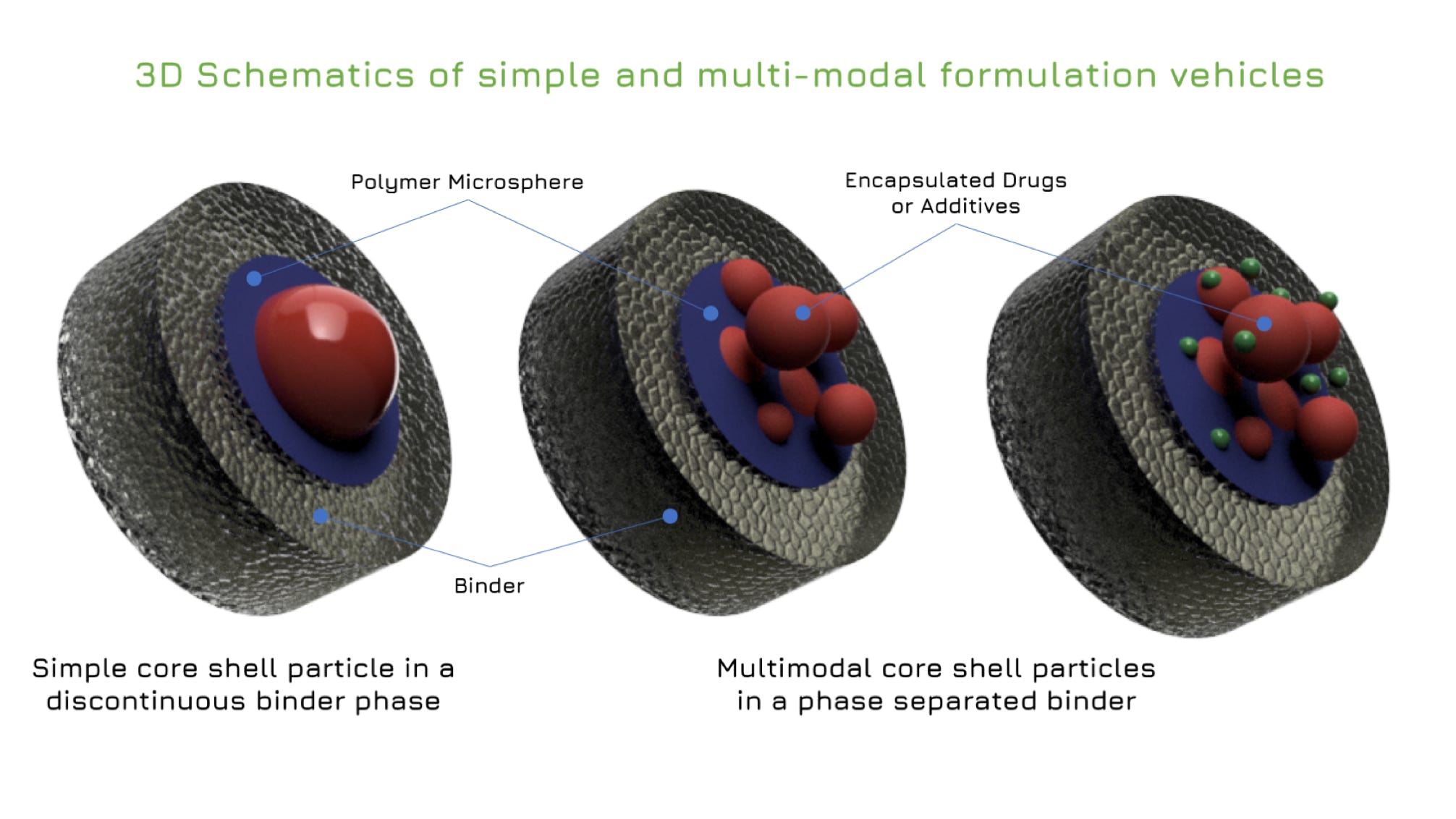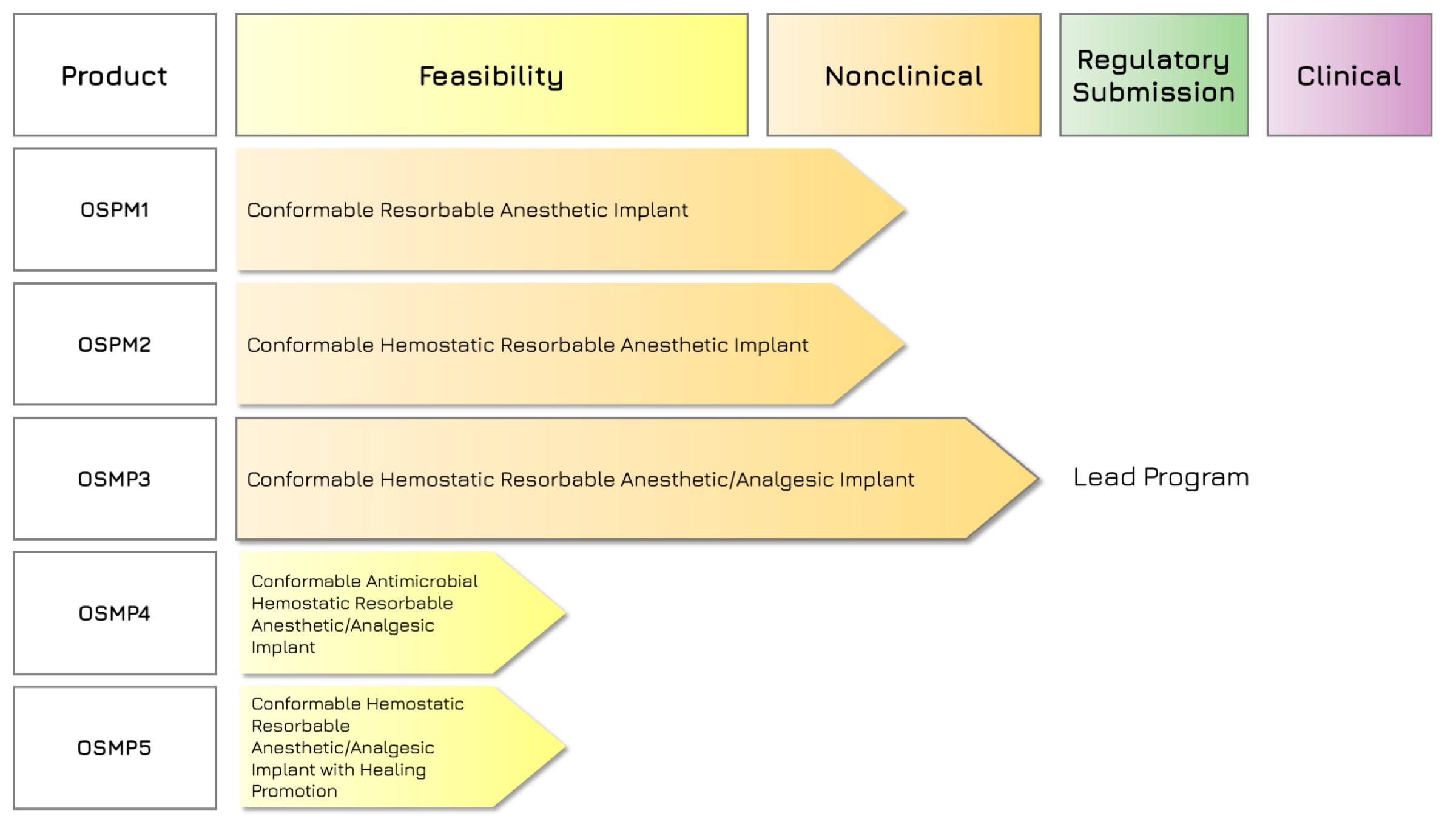
Rilento is developing innovative drug delivery systems designed to manage pain after oral surgery. By using the FDA's 505(b)(2) regulatory pathway, Rilento can leverage existing knowledge of approved drugs to streamline the approval process. This approach allows them to efficiently demonstrate the effectiveness of their systems in treating not only oral surgery pain, but potentially other types of surgical and injury-related pain as well. Ultimately, Rilento aims to provide a preventative pain management strategy that reduces the need for opioid-based medications.
Rilento's drug delivery systems are designed to tackle pain after oral surgery by combining different medications for maximum effect. These systems can deliver an anesthetic alone, or pair it with anti-inflammatory painkillers that reduce swelling and pain by blocking COX1 and COX2 enzymes. This dual action helps prevent the initial pain from turning into a more sensitive, long-lasting pain state. By including a long-acting anesthetic, the system provides continuous pain relief for up to 72 hours after surgery. This extended release, combined with the anti-inflammatory action, aims to minimize pain and help patients regain normal function quickly after their procedure.
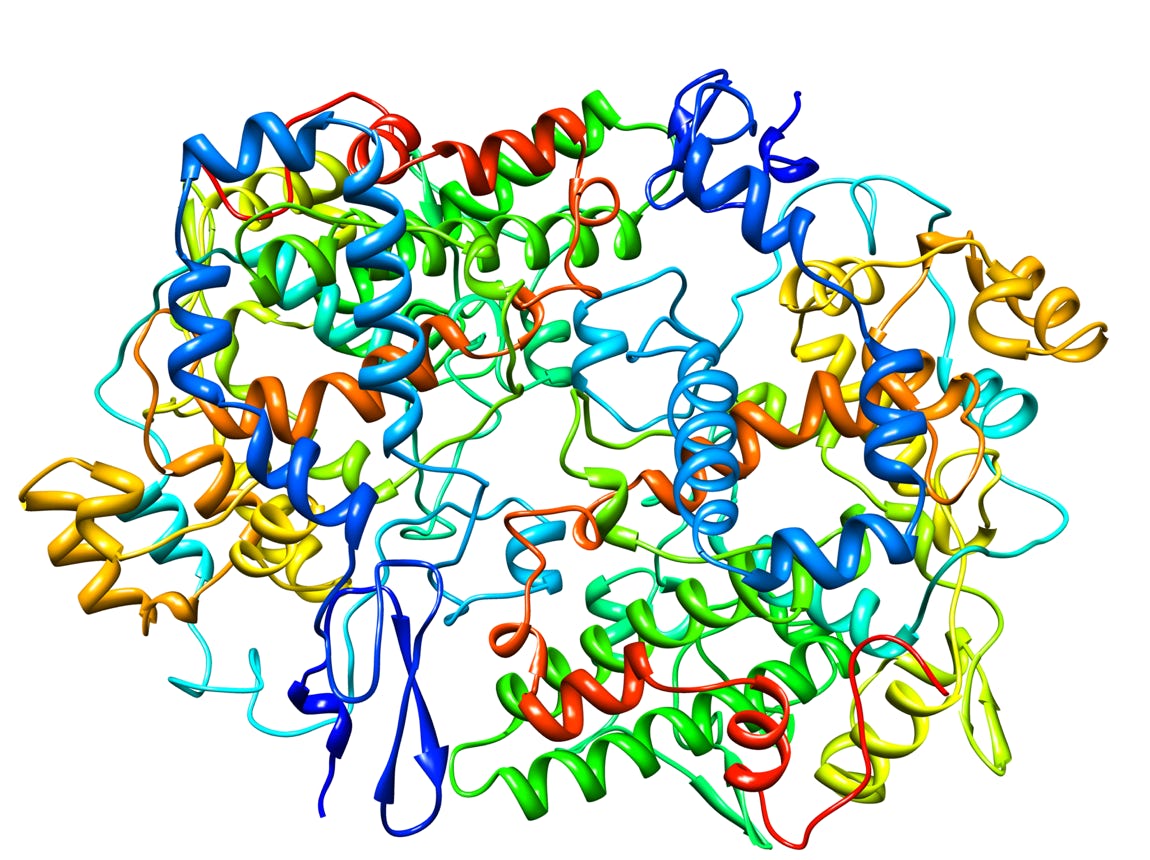
Cyclooxygenase-1 (Prostaglandin Synthase-1) in complex with a COX-1 selective inhibitor (1)
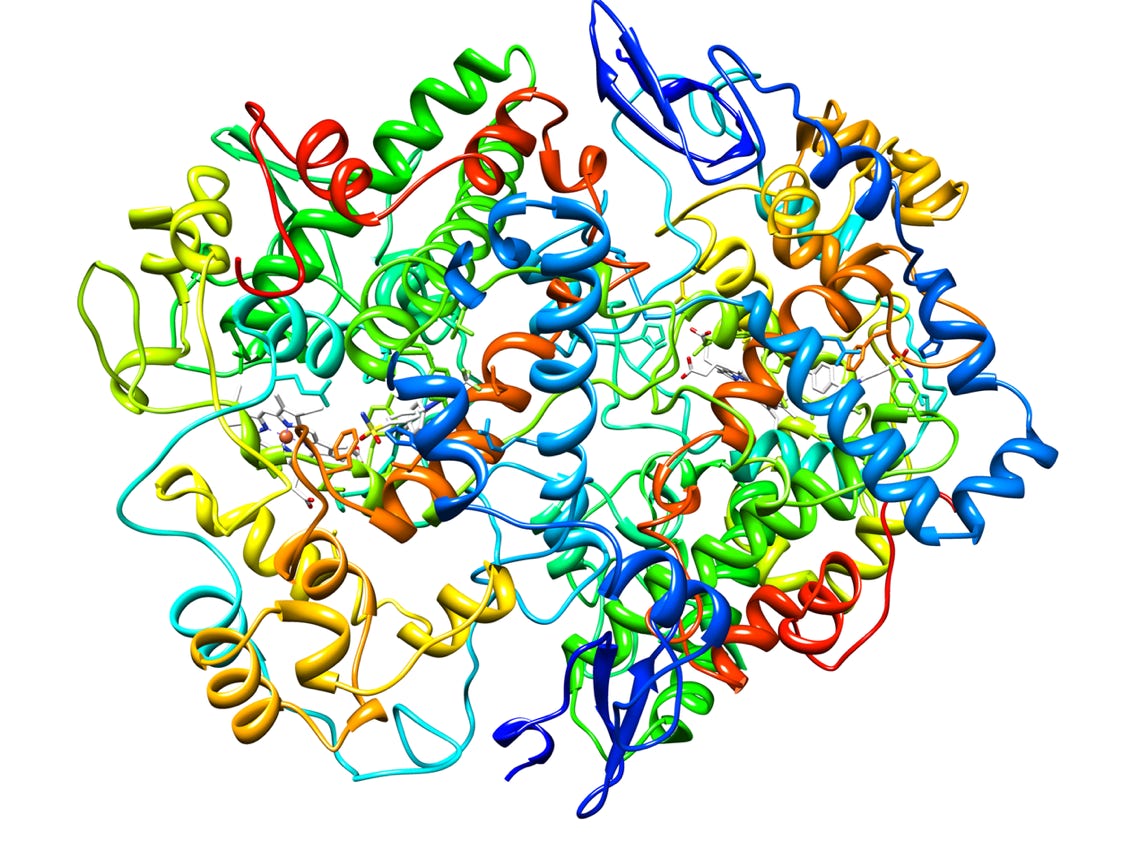
Cyclooxygenase-2 (Prostaglandin Synthase-2) in complex with a COX-2 selective inhibitor (2)